Products
Sincalide for Injection
First-to-Market Authorized
Generic of Kinevac®*
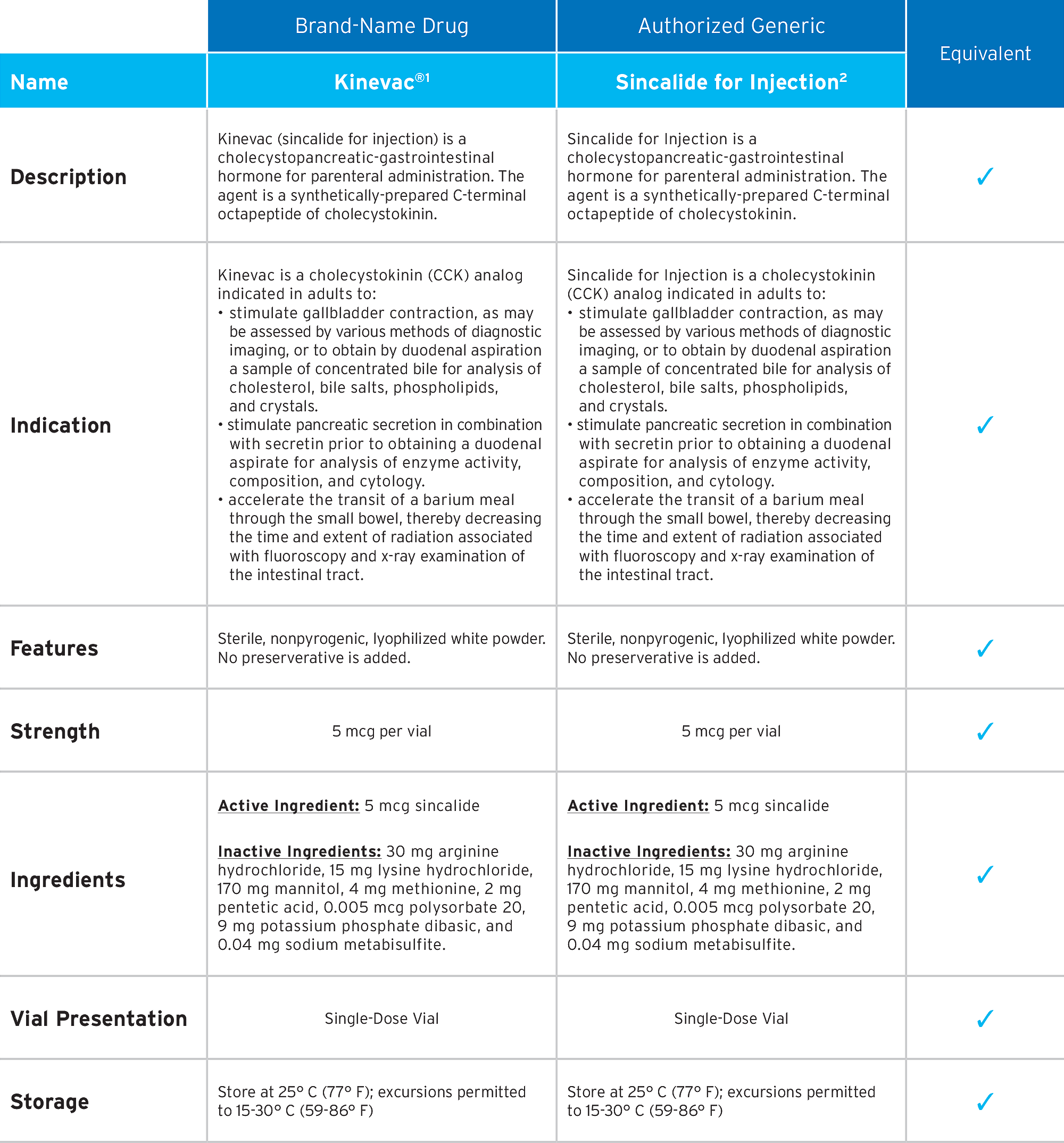
Fresenius Kabi’s Sincalide for Injection is the first-to-market authorized generic of Kinevac®.* Sincalide for Injection meets the same quality standards as the branded product, while providing you with choice and value.
*Kinevac is a registered trademark of Bracco Diagnostics Inc. Please see Full Prescribing Information and Important Safety Information for Kinevac injection. Download the Kinevac Safety Data Sheet (SDS).
1. Kinevac Prescribing Information, November 2023
2. Sincalide for Injection Prescribing Information, October 2023
Sincalide for Injection
• Authorized Generic of Kinevac®*
• Preservative Free
• Container closure is not made with natural rubber latex

*Kinevac is a registered trademark of Bracco Diagnostics Inc.
Downloads
Sincalide for Injection Brochure
Download
Important Safety Information
Contraindications
Sincalide for Injection is contraindicated in patients with a history of hypersensitivity to sincalide, including anaphylaxis and anaphylactic shock, or in patients with intestinal obstruction.
Warnings and Precautions
Post-marketing anaphylaxis, anaphylactic shock, and other serious hypersensitivity reactions have been reported during and within one hour following administration of sincalide. If anaphylaxis or other hypersensitivity reactions occur, immediately discontinue the infusion and initiate appropriate medical treatment.
Stimulation of gallbladder contraction can lead to small gallbladder stone evacuation, resulting in lodging in the cystic duct or in the common bile duct.
Sincalide may cause adverse reactions such as nausea, vomiting, abdominal pain or cramping, dizziness, and flushing. To reduce the risk of adverse reactions, administer sincalide over 50 minutes for simulation of gallbladder contraction or over 30 minutes to accelerate transit of a barium meal through the small intestine.
Pregnant patients should be advised that sincalide can effect smooth muscle, which may cause spontaneous abortion or premature induction of labor.
The most common adverse reactions (≥ 20%) include abdominal discomfort or pain, and nausea.
INDICATIONS
Sincalide for Injection is indicated in adults to:
- stimulate gallbladder contraction, as may be assessed by various methods of diagnostic imaging, or to obtain by duodenal aspiration a sample of concentrated bile for analysis of cholesterol, bile salts, phospholipids, and crystals
- stimulate pancreatic secretion (especially in conjunction with secretin) prior to obtaining a duodenal aspirate for analysis of enzyme activity, composition, and cytology
- accelerate the transit of a barium meal through the small bowel, thereby decreasing the time and extent of radiation associated with fluoroscopy and x-ray examination of the intestinal tract
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for Sincalide for Injection.
©2024 Fresenius Kabi USA, LLC. All Rights Reserved. 2589-IODX-08-03/24
©2024 Bracco Diagnostics Inc. All Rights Reserved. US-SN-2300007 03/24


